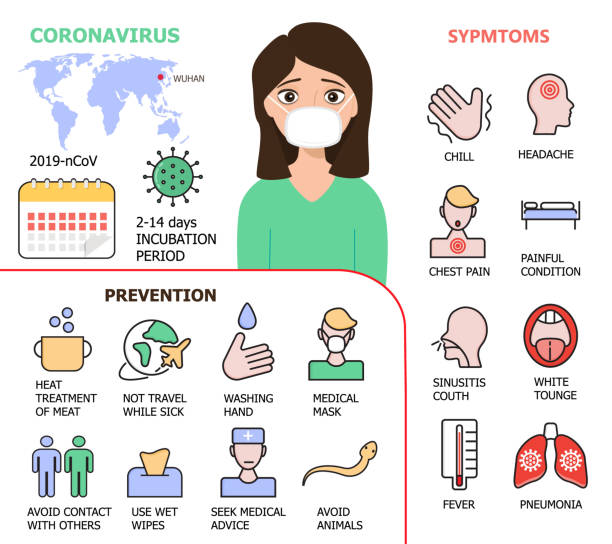
Title: Medicine for Coronavirus: Advancements, Challenges, and Hope
In the wake of the global pandemic that emerged in late 2019, scientists and researchers around the world embarked on a race against time to find effective medicine for the novel coronavirus, SARS-CoV-2. The urgent need to alleviate the immense human suffering and loss prompted an unprecedented level of collaboration, innovation, and dedication in the field of medical science. As we delve into the ongoing efforts to develop medicines for coronavirus, we witness a narrative of both advancements and challenges, underscoring the delicate balance between scientific progress and the complexities of a rapidly evolving virus.
Advancements in Medicine Development:
The journey to find a definitive medicine for coronavirus has seen remarkable scientific achievements. One of the most significant breakthroughs has been the development of vaccines, which are essential tools in controlling the spread of the virus and reducing the severity of illness. Vaccines such as Pfizer-BioNTech, Moderna, AstraZeneca, and Johnson & Johnson have demonstrated their efficacy in large-scale clinical trials. These vaccines have not only played a pivotal role in curbing the transmission of the virus but have also paved the way for further research in the field of immunology.
In addition to vaccines, antiviral medicines have also garnered attention. Remdesivir, initially developed for Ebola, gained Emergency Use Authorization as a treatment for COVID-19. While not a panacea, remdesivir has shown promise in reducing the recovery time for hospitalized patients. Monoclonal antibody therapies, such as casirivimab and imdevimab, have been authorized for emergency use to treat mild to moderate cases, particularly in high-risk individuals. These advancements highlight the adaptability of medical research in repurposing existing treatments to address emerging challenges.
Challenges on the Road to Recovery:
While progress has been made, several challenges persist in the pursuit of effective medicines for coronavirus. One major hurdle is the rapid mutation rate of the virus. Variants of concern, such as the Delta variant, have raised concerns about vaccine efficacy and the potential for reduced effectiveness of existing treatments. Researchers must continuously monitor and adapt their strategies to stay ahead of the evolving virus.
Clinical trials also pose challenges. Conducting rigorous trials while maintaining ethical standards and expediting the process requires a delicate balance. The urgency of the pandemic has led to accelerated trial timelines, but thorough assessment of safety and efficacy remains paramount. Balancing the need for speed with the need for robust data is an ongoing challenge that researchers grapple with.
The Path Forward:
Despite the challenges, the medical community remains steadfast in its commitment to finding effective medicines for coronavirus. A holistic approach that combines vaccines, antiviral drugs, and supportive care is likely to yield the most comprehensive results. Continued investment in research infrastructure, international collaboration, and data sharing will be crucial in the fight against COVID-19 and potential future pandemics.
Advancements in technology also offer hope. Artificial intelligence and machine learning are being employed to accelerate drug discovery and predict potential drug interactions. These tools can sift through vast amounts of data to identify promising compounds and shorten the timeline for development.
In conclusion, the pursuit of medicines for coronavirus stands as a testament to human resilience and determination. The remarkable advancements achieved in vaccine development and repurposing existing treatments have provided valuable tools in combating the pandemic. However, challenges such as the virus's mutational nature and the complexities of clinical trials remind us of the intricate nature of medical research. As we navigate this intricate landscape, it is imperative that we continue to foster collaboration, embrace innovation, and remain adaptable in the face of uncertainty. The story of medicine for coronavirus is not only one of scientific progress but also of the unwavering human spirit in the face of adversity.
In the wake of the global pandemic that emerged in late 2019, scientists and researchers around the world embarked on a race against time to find effective medicine for the novel coronavirus, SARS-CoV-2. The urgent need to alleviate the immense human suffering and loss prompted an unprecedented level of collaboration, innovation, and dedication in the field of medical science. As we delve into the ongoing efforts to develop medicines for coronavirus, we witness a narrative of both advancements and challenges, underscoring the delicate balance between scientific progress and the complexities of a rapidly evolving virus.
Advancements in Medicine Development:
The journey to find a definitive medicine for coronavirus has seen remarkable scientific achievements. One of the most significant breakthroughs has been the development of vaccines, which are essential tools in controlling the spread of the virus and reducing the severity of illness. Vaccines such as Pfizer-BioNTech, Moderna, AstraZeneca, and Johnson & Johnson have demonstrated their efficacy in large-scale clinical trials. These vaccines have not only played a pivotal role in curbing the transmission of the virus but have also paved the way for further research in the field of immunology.
In addition to vaccines, antiviral medicines have also garnered attention. Remdesivir, initially developed for Ebola, gained Emergency Use Authorization as a treatment for COVID-19. While not a panacea, remdesivir has shown promise in reducing the recovery time for hospitalized patients. Monoclonal antibody therapies, such as casirivimab and imdevimab, have been authorized for emergency use to treat mild to moderate cases, particularly in high-risk individuals. These advancements highlight the adaptability of medical research in repurposing existing treatments to address emerging challenges.
Challenges on the Road to Recovery:
While progress has been made, several challenges persist in the pursuit of effective medicines for coronavirus. One major hurdle is the rapid mutation rate of the virus. Variants of concern, such as the Delta variant, have raised concerns about vaccine efficacy and the potential for reduced effectiveness of existing treatments. Researchers must continuously monitor and adapt their strategies to stay ahead of the evolving virus.
Clinical trials also pose challenges. Conducting rigorous trials while maintaining ethical standards and expediting the process requires a delicate balance. The urgency of the pandemic has led to accelerated trial timelines, but thorough assessment of safety and efficacy remains paramount. Balancing the need for speed with the need for robust data is an ongoing challenge that researchers grapple with.
The Path Forward:
Despite the challenges, the medical community remains steadfast in its commitment to finding effective medicines for coronavirus. A holistic approach that combines vaccines, antiviral drugs, and supportive care is likely to yield the most comprehensive results. Continued investment in research infrastructure, international collaboration, and data sharing will be crucial in the fight against COVID-19 and potential future pandemics.
Advancements in technology also offer hope. Artificial intelligence and machine learning are being employed to accelerate drug discovery and predict potential drug interactions. These tools can sift through vast amounts of data to identify promising compounds and shorten the timeline for development.
In conclusion, the pursuit of medicines for coronavirus stands as a testament to human resilience and determination. The remarkable advancements achieved in vaccine development and repurposing existing treatments have provided valuable tools in combating the pandemic. However, challenges such as the virus's mutational nature and the complexities of clinical trials remind us of the intricate nature of medical research. As we navigate this intricate landscape, it is imperative that we continue to foster collaboration, embrace innovation, and remain adaptable in the face of uncertainty. The story of medicine for coronavirus is not only one of scientific progress but also of the unwavering human spirit in the face of adversity.